Long-term efficacy and safety of siponimod in patients with secondary progressive multiple sclerosis: Analysis of EXPAND core and extension data up to >5 years - Bruce AC Cree, Douglas L Arnold, Robert

Phase 1 trial of olaratumab monotherapy and in combination with chemotherapy in pediatric patients with relapsed/refractory solid and central nervous system tumors - Mascarenhas - 2021 - Cancer Medicine - Wiley Online Library

A randomized clinical trial on the short‐term effects of 12‐week sacubitril/valsartan vs. enalapril on peak oxygen consumption in patients with heart failure with reduced ejection fraction: results from the ACTIVITY‐HF study -
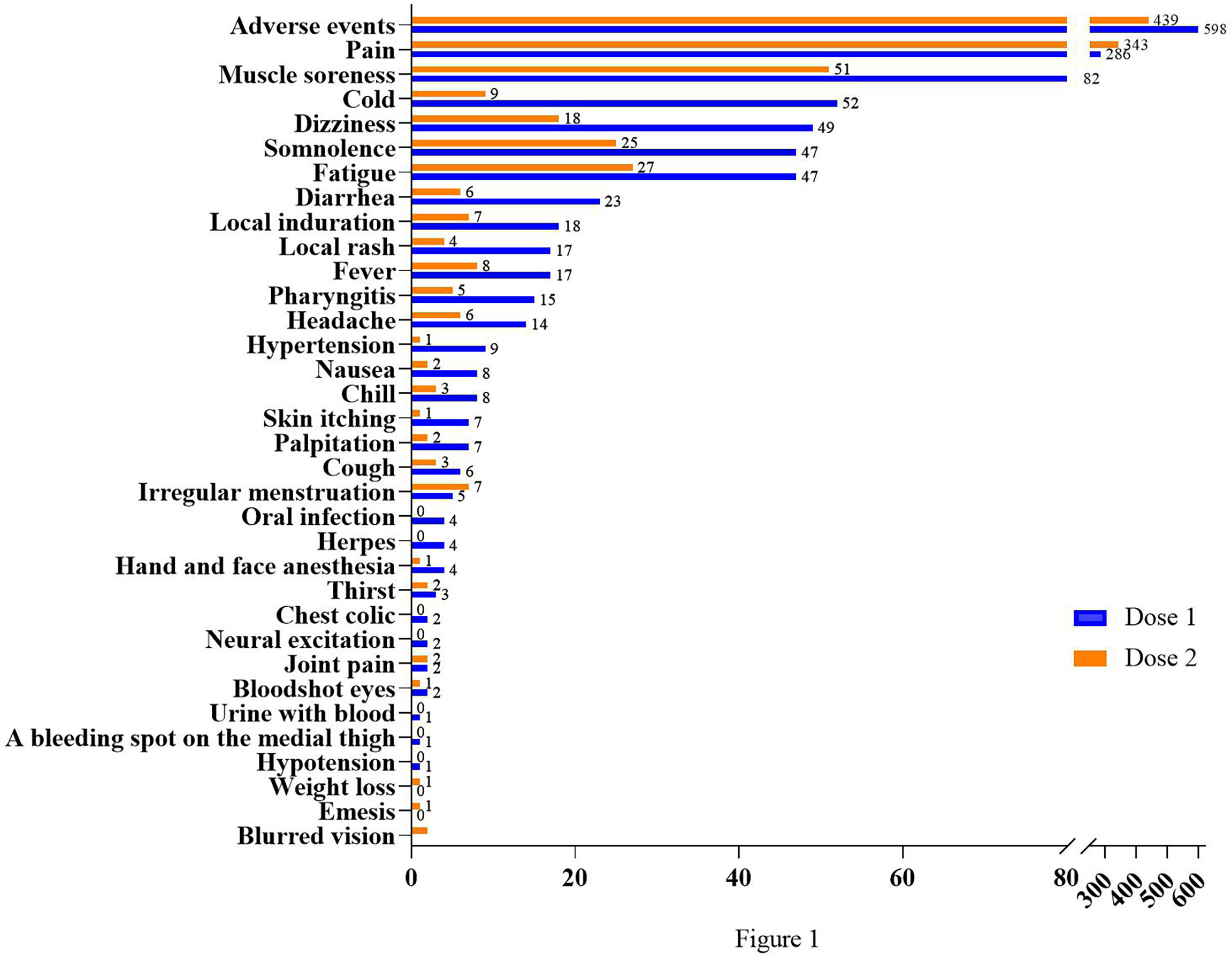
Frontiers | Safety monitoring in inactivated COVID-19 vaccines by clinical pharmacists from a single center in China

Association of Total Medication Burden With Intensive and Standard Blood Pressure Control and Clinical Outcomes: A Secondary Analysis of SPRINT | Hypertension

A Phase 3, Multicenter, Randomized, Controlled Trial to Evaluate Immune Equivalence and Safety of Multidose and Single-dose Formulations of Vi-DT Typhoid Conjugate Vaccine in Healthy Filipino Individuals 6 Months to 45 Years
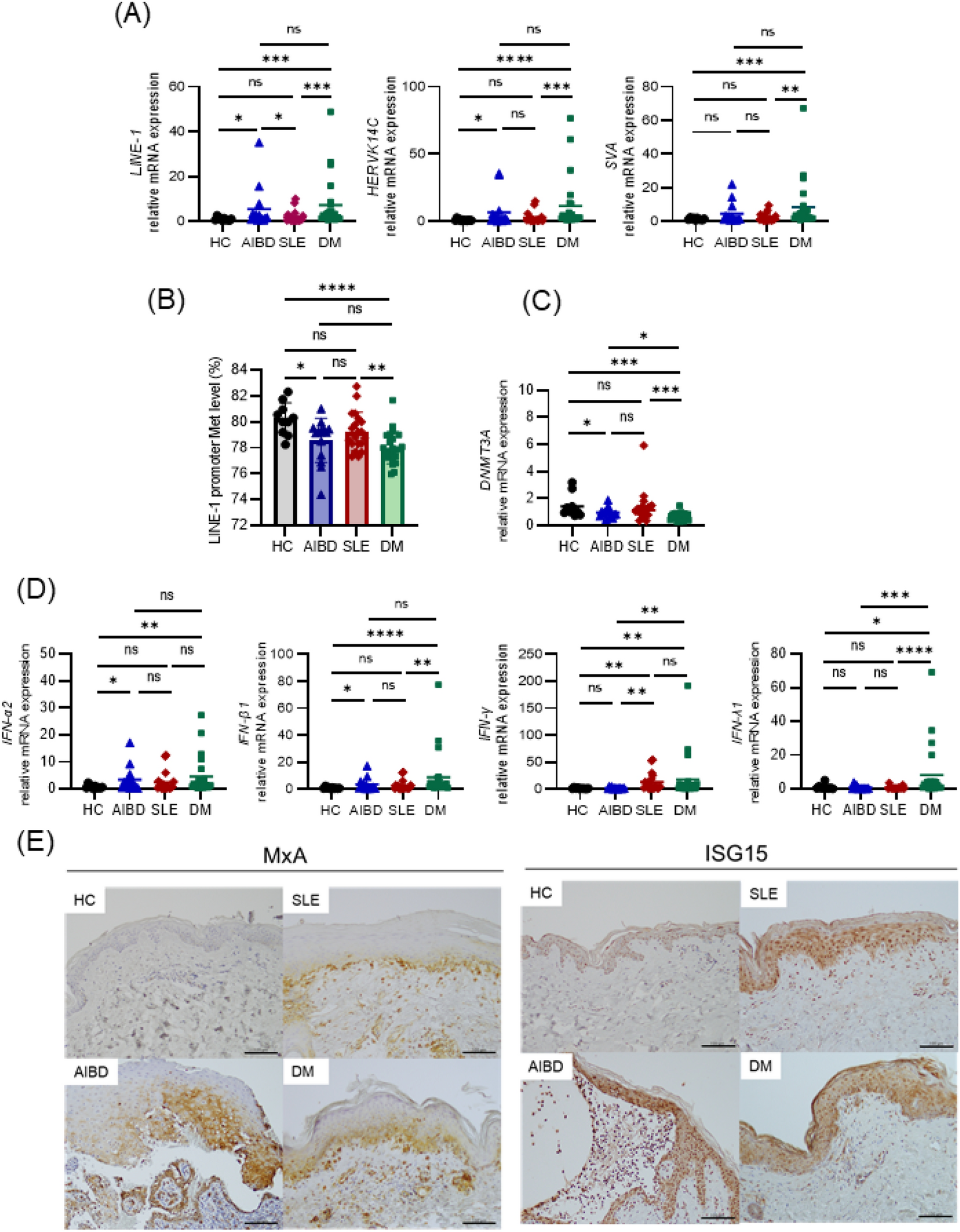
Coordination of retrotransposons and type I interferon with distinct interferon pathways in dermatomyositis, systemic lupus erythematosus and autoimmune blistering disease | Scientific Reports
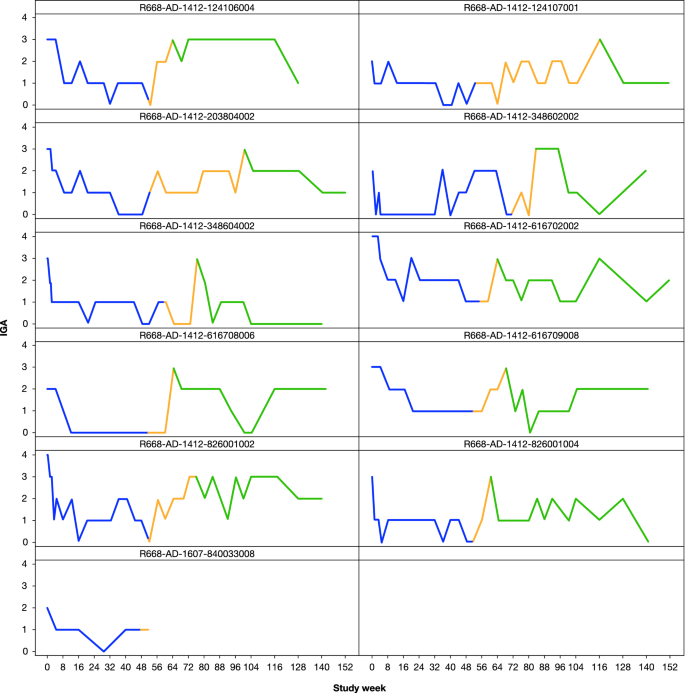
Long-Term Efficacy and Safety of Dupilumab in Adolescents with Moderate-to-Severe Atopic Dermatitis: Results Through Week 52 from a Phase III Open-Label Extension Trial (LIBERTY AD PED-OLE) | SpringerLink

Safety and immunogenicity of Vi-DT conjugate vaccine among 6-23-month-old children: Phase II, randomized, dose-scheduling, observer-blind Study - eClinicalMedicine
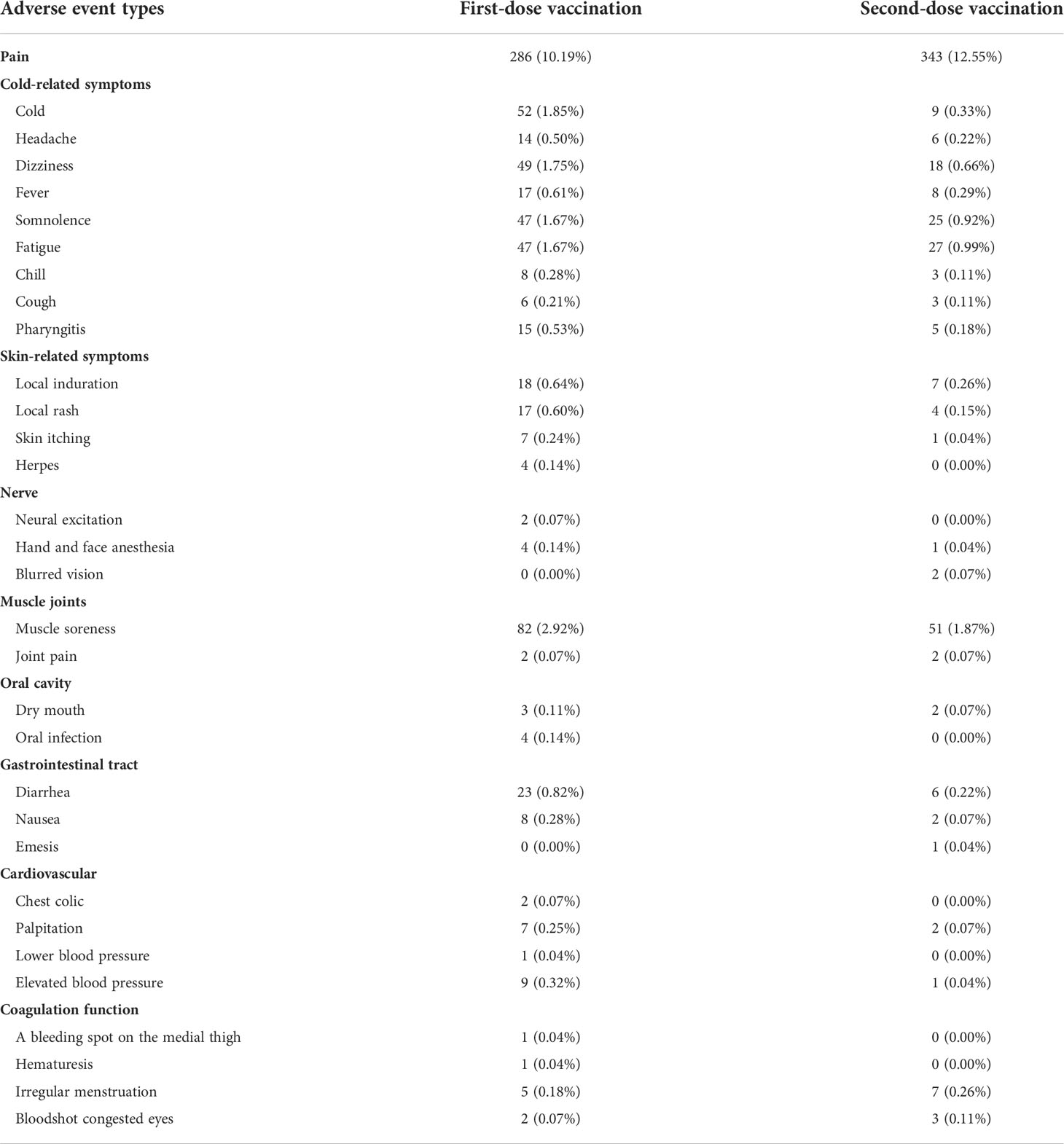
Frontiers | Safety monitoring in inactivated COVID-19 vaccines by clinical pharmacists from a single center in China

Fatty Acid Metabolites Combine with Reduced β Oxidation to Activate Th17 Inflammation in Human Type 2 Diabetes - ScienceDirect

Association of Total Medication Burden With Intensive and Standard Blood Pressure Control and Clinical Outcomes: A Secondary Analysis of SPRINT | Hypertension
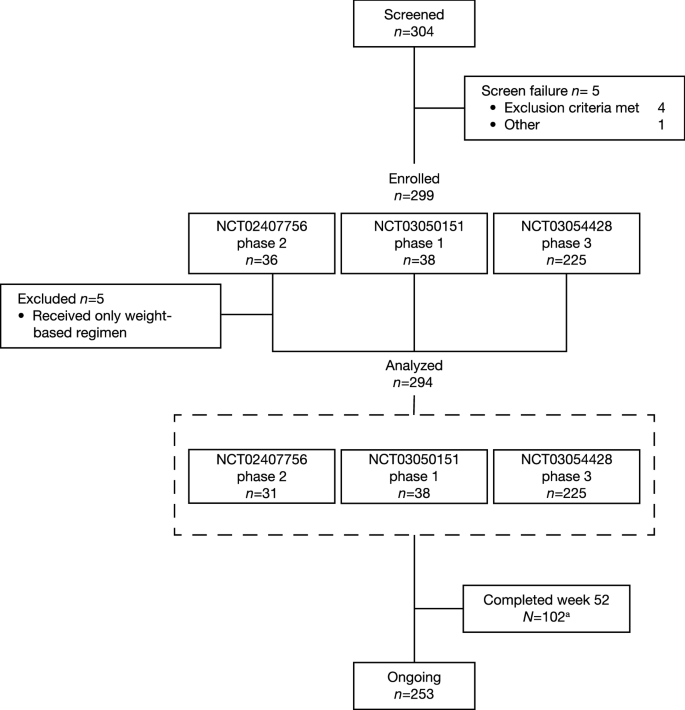
Long-Term Efficacy and Safety of Dupilumab in Adolescents with Moderate-to-Severe Atopic Dermatitis: Results Through Week 52 from a Phase III Open-Label Extension Trial (LIBERTY AD PED-OLE) | SpringerLink
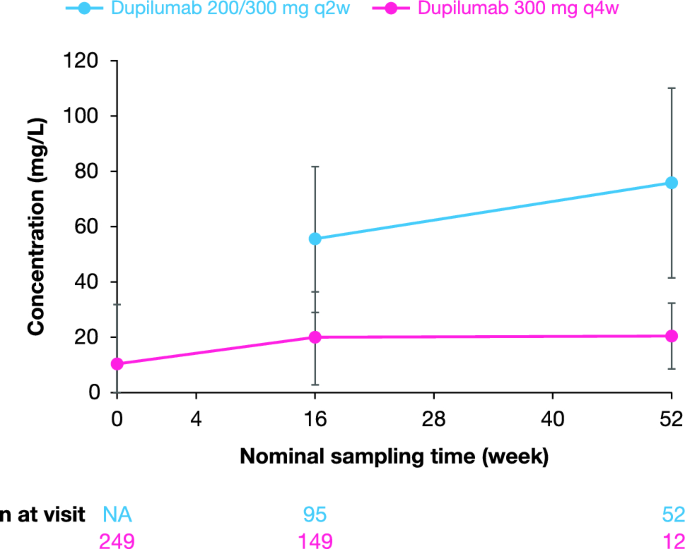
Long-Term Efficacy and Safety of Dupilumab in Adolescents with Moderate-to-Severe Atopic Dermatitis: Results Through Week 52 from a Phase III Open-Label Extension Trial (LIBERTY AD PED-OLE) | SpringerLink

Dupilumab provides favourable long‐term safety and efficacy in children aged ≥ 6 to < 12 years with uncontrolled severe atopic dermatitis: results from an open‐label phase IIa study and subsequent phase III
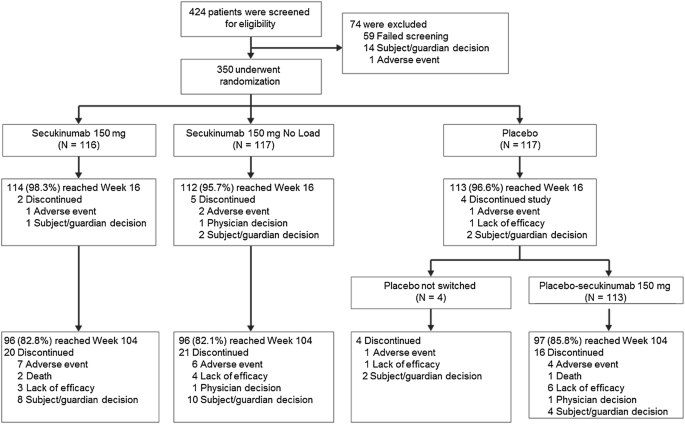
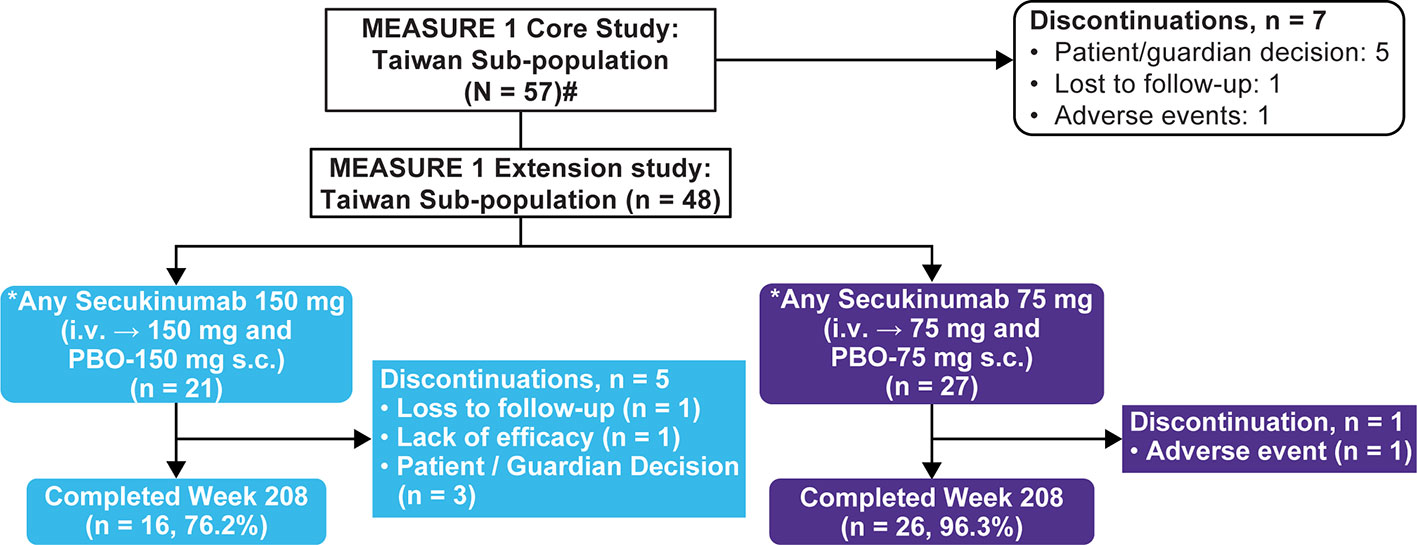
Efficacy and Safety of Secukinumab 150 mg with and Without Loading Regimen in Ankylosing Spondylitis: 104-week Results from MEASURE 4 Study | SpringerLink
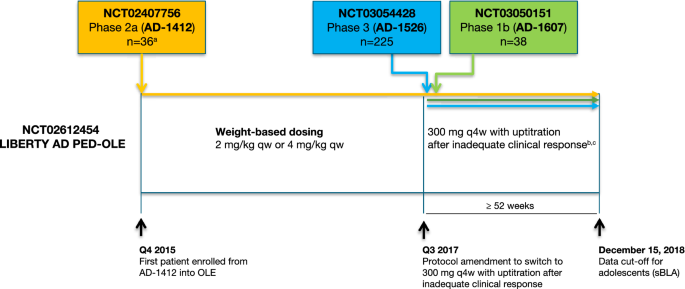
Long-Term Efficacy and Safety of Dupilumab in Adolescents with Moderate-to-Severe Atopic Dermatitis: Results Through Week 52 from a Phase III Open-Label Extension Trial (LIBERTY AD PED-OLE) | SpringerLink